
Translation Agency | Email US | Translation Resources | Translation Jobs | Translation Agencies
World Languages | Translation Tips | Translation Services
Translations of Encyclopedia by Czech and Slovak to English translator

Translation Agency |
Email US |
Translation
Resources | Translation Jobs | Translation Agencies
World Languages | Translation Tips | Translation Services
AlcoholsThere are a number of homologous series of hydrocarbon compounds which contain various functional groups that are derived from alkanes, alkenes, alkynes and benzene. Alcohols are hydrocarbons which contain the hydroxyl group, an -OH group, as their functional group. Alcohols are named from the corresponding molecule they derive from, then given an -ol ending, which describes the -OH functional group. The simplest alcohols are methanol (CH3OH) and ethanol (CH3CH2OH). Cyclic hydrocarbons can also form alcohols.Phenol is derived from benzene, its hydroxylic functional group joining onto the aromatic ring. Alcohols with multiple hydroxylic groups are called multiple hydroxyl-containing alcohols. The most meaningful of these are ethylene glycol and propantriol, otherwise known as glycerine. Alcohols are flammable, burning to produce carbon dioxide and water. The chemical properties of alcohols are quite similar to each other, thanks to the presence of the hydroxyl functional group. Alcohols derived from alkanes form alcoholates with impure metals. In these reactions, the hydrogen atom from the hydroxyl group is exchanged with a metal atom. This is called a substitution reaction.CH 2OH-CHOH-CH2OHglycerine propantriol A sizable number of compounds which appear in living organisms contain a significant number of hydroxylic groups. Among these are simple sugars such as glucose or fructose, a sugar derived from fruits, as well as some hormones (steroidal hormones).Structure of the molecule and functional group The functional group determines to a great degree the chemical properties of an organic material. In addition, the placement of the functional group can also have a great influence on a molecule’s chemical properties. There are three distinct types of alcohols, their grouping based on where the functional group is placed in the molecule. Primary alcohols have their -OH functional group bonded to one atom of carbon which is in turn bonded to only one other carbon atom. Methanol and ethanol are primary alcohols. Just as the bond between oxygen and hydrogen is polar, so is the bond between carbon and oxygen polar. Thanks to the differing electronegativities of these elements, they have a different attraction to the electron pair which is formed between them. Oxygen, because of its higher electronegativity, attracts the electrons more strongly than carbon. For this reason, and because it contains another two pairs of electrons, a partial negative charge is present on the oxygen atom. These charges make alcohols polar molecules. |
|
+
-Ethanol CH
Chain alcohols have their simple bonds derived from corresponding alkanes. For this reason, we call this type of alcohol aliphatic. These alcohols form a homologous series whose members have the general formula CnH2n+1OH. The name of these alcohols comes from the corresponding alkane plus the suffix -ol. Just as the alkanes, isomeres exist in this alcohol series, beginning with propanol. The placement of the hydroxyl group is indicated by a numbering system which pinpoints the carbon to which the group is bonded, with that number placed either before or after the name of the molecule.
The oxygen atom strongly attracts the electron pair it shares with carbon. Combined with two other electron pairs already found on the oxygen atom, the oxygen represents a partial negative charge compared to the rest of the molecule. The hydroxylic group is hydrophilic, meaning that it attracts water. The rest of the molecule, the hydrocarbon chain, is hydrophobic, meaning that it repels water.
CH
3-CH2-CH2-CH2-CH2-O-HHydrophobic Rest Hydrophilic Group
The hydrophobic character of the hydrocarbon chain increases as the chain gets longer. Within the hydrophobic regions of individual molecules are
van der Waals forces which hold those regions together. Alkane-derived alcohols become more viscous as the length of their chains increases, because forces within the molecule become more and more exaggerated. Solubility in water decreases as molecule size grows. The hydrophilic hydroxyl group of the molecule forms hydrogen bonds with other neighbouring hydroxyl groups. These hydrogen bonds are stronger than normal van der Waals forces, and they influence the melting and boiling points of alcohols. The melting and boiling points of alcohols, therefore, are significantly higher than those of alkanes.|
The production of ethanol Ethanol is the most significant and important member of the alcohol family derived from alkanes. Various processes which result in the production of alcohol resulting from fermented materials are some of the best known chemical processes, and one of the very best known is the production of wine. In this process, varying degrees of ethanol are produced from the alcoholic fermentation of sugars, in the presence of enzymes. A by-product of the reaction is carbon dioxide. This type of alcoholic fermentation results in a mixture which has a 10-14 % alcoholic content. Ethanol can also be produced synthetically for certain industrial uses. When this type of process takes place, the hydroxylic group from water is added onto ethane, in the presence of a catalyst.Ethanol is a clear colourless liquid with a characteristic odour. It is strongly hydroscopic (attracts water), and can be mixed with water in an unlimited fashion. Ethanol is a flammable liquid. When it does burn, carbon dioxide and water are produced. It denatures proteins and dissolves a number of organic substances. For this reason, it is preferred as a solvent in industry rather than in the household. It is also an ingredient in a number of cosmetic and medicinal products. In the household, denatured alcohol, to which is added bits of other materials, is used as a combustible and a cleaning product. In some countries, ethanol produced from plant matter is used as a fuel mixed with petrol. The use of ethanol as an additive to some grocery products (drinking alcohol) is very widespread. The use of alcohol first causes blood circulation in the body to increase depending on the dose taken. With increasing dose size, however, both physical and mental faculties can be hindered. This can does often lead to permanent damage. Long-term use can result in permanent damage to some vital internal organs which play large roles in the circular system, as well as the liver and the brain. This damage is the result of ethanol’s denaturing and dissolving properties. Ethanol is considered to be a habit-forming substance. There are more human beings addicted to ethanol than to the so-called harder drugs. Structure of the molecule and its reactions Ethanol, derived from ethane, contains a functional hydroxyl group bonded to one atom of carbon which is in turn bonded to another atom of carbon. Ethanol, therefore, is categorised as a primary alcohol. The reactivity of ethanol is to a great degree determined by the presence of its functional group. Ethanol reacts with impure metals to produce ethyl metals. In this type of reaction, the hydrogen atom of the hydroxyl group is replaced by an atom of metal in a substitution reaction. 2CH 3-CH2-OH + 2 Na ® 2 CH3-CH2-ONa + H2ethanol sodiumethanolate |
|
Phenol
Phenol is a hydroxylic derivative of benzene. It easily forms regular crystals (
J S = 43° C) which turn red when exposed to air. It has a distinctive odour. Compared with other alcohols, phenol does not dissolve in water as readily. It does, however, mix with ethanol relatively easily. In an aqueous solution, phenol reacts significantly more acidicly than other alcohols. It is poisonous and corrosive.The hydroxyl group in a molecule of phenol is bonded to a carbon on the inner ring, so the systematic name of phenol is monohydroxybenzol. The electrons of the non-bonding electron pairs in the ring
come into interaction with the electrons of the oxygen in the hydroxylic group, increasing the density of the electrons in the ring. This phenomenon is known as the positive mesomeric effect (+M). The result of this effect is that the bonding electrons of the bonds between oxygen and hydrogen are more strongly attracted to the oxygen atom, making the bond between the two a polar one. This leads to the hydrogen atom of the hydroxyl group being easily dissociated, thus forming the phenyl ion. Because phenol is able to dissociate the proton on its hydroxyl group rather easily, is can be used in solution to indicate, or recognise, an acid. Phenol is one of the reactants used in the production of plastics, paints and coatings, and herbicides.
Methanol
Methanol is the first member of the homologous series of alkane-derived alcohols. It has similar chemical properties to ethanol. Methanol is, of course, highly toxic. Its use can lead to blindness, and in higher dosages of around 50 ml, even to death. Methanol is a clear, colourless liquid with a characteristic odour. In industry, methanol is used in the production of formaldehyde,
esters and acetic acid.Translating Bulgarian Dutch Translations Dutch Bulgarian Translating Polish Ukrainian Translations Ukrainian Polish Translating Norwegian
Bicarboxylic acids
Bicarboxylic acids have two carboxylic groups. Many bicarboxylic compounds are biologically very important.
Oxalic acid (HOOC-COOH) is found in most types of vinegars. .
Amber acid (HOOC-CH
2-CH2-COOH) is found in living organisms. It helps in material exchange of some of the important materials organisms need. With its help, a process known as biological oxidation (cell breathing) takes place. This is one of the most important biological processes, one in which the body synthesises its own energy.Adipatic acid (HOOC-(CH
2)4-COOH)Hydroxycarboxylic acids
In molecules of carboxylic acids, one or more
hydrogen atoms in the hydrocarbon chain can be exchanged for a hydroxylic group (-OH). Hydroxycarboxylic acids are often found in nature and are important because they take part in a number of important biological processes. These carboxylic acids, because of their nature, used to be called plant acids.Important hydroxycarboxylic acids
Lactic acid CH
3-CH-COOH˝
OH
Many microorganisms breakdown
hydrocarbons to produce lactic acid. Lactic sugar is found in milk, and this is also easily broken down to produce lactic acid. Thanks to the acidic atmosphere in which this degradation takes place, a layer of agglutinated proteins forms on the top surface of the milk. This is cream. Lactic acid is also produced when acidic cabbage, cucumbers or silage are fermented.Lactic acid is produced in muscle tissue, too - when glycogens are broken down and not enough oxygen is present. This lack of oxygen is responsible for the pain we feel in muscle tissue, and is also a sign of fatigue.
Wine acid? HOOC-CH-CH-COOH
˝ ˝
OH OH
In plants, this type of so-called wine acid occurs as a component of some vines, especially grape vines. It is also one of the most important compounds in wine, along with potassium, vinny kamen.
Vinný kámen is a material used in the textile industry, finding its significance in the dying or colouring of some materials. Another compound, hydrogentartarate draselny, with the common name baking powder, is used in the household.

A number of plant acids can be found in the bodies of living organisms. They are important as intermediate products in the decomposition of hydrocarbons. They are also used in the production of some sweets, syrups and textiles.

A lack of vitamin C can cause scurvy (a disease caused by a lack of vitamins that can be fatal if not treated). Ascorbic acid helps to slow down uncontrolled oxidation, because it oxidises more quickly as a replacement. Human beings need around 75 mg of vitamin C per day. It is found in some plant material, including fruits and vegetables. Otherwise, ascorbic acid is used as a preservative.
Salicylic acid
Salicylic acid is used as a preservative, too. A compound it forms with sodium, sodium salicylate, is a very good medicine used for rheumatic diseases. It is also the base material contained in aspirin.
Translating Czech Italian Translations Italian Czech Translating Ukrainian Norwegian Translations Norwegian Ukrainian Translating Finnish
|
Éthers are composed of two hydrocarbon groups which are bonded to each other by means of an atom of oxygen. The oxygen centre does not influence the arrangement or characteristics of the ether. The properties of ethers are similar to the properties of other hydrocarbons, and especially alkanes. Ethers, however, are not capable of bonding with hydrogen atoms. Ethers form isomeric compounds with alcohols. For example, ethyl alcohol (CH3CH2OH) has the same chemical formula as dimethylether (CH3OCH3). This compound is the best known and most often mentioned of all of the ether family.Dimethylether has a low boiling point. It is very explosive and flammable. Ethers are very soluble and were often used as narcotics to help people fall asleep in past times. The ether compound (C-O-C) is common in nature, often being found in sugars, cellulose and lignite.Aldehydes and Ketones Aldehydes and ketones are among the compounds which contain oxygen, the bonds they form with oxygen being characteristic for these types of compounds. Aldehydes are derived from the primary alcohols. They contain the so-called aldehyde group in their molecule. The aldehyde group is one atom of carbon which is double bonded to an atom of oxygen and single bonded to an atom of hydrogen. Aldehydes are named according to the corresponding alkane they are derived from, with an -al ending at the end. The first group members have traditional names. The homologous group begins with formaldehyde. Formaldehyde H – C = O ˝ H The functional group is very reactive and has reductive characteristics. Aldeydes reduce silver ions to silver metal, as well as reducing copper ions with a plus 2 charge to copper ions with just one positive charge. Formaldehyde is a found in the gaseous state of matter. It is used as a 38% solution (formalin). |
|
It is produced in quantity by the catalytic oxidation of methanol in the presence of and with the help of oxygen. Formaldehyde is a reactant in the process of the chemical production of plastic materials and other products. In the form of its solution, formalin it is used as a preservative. Because of its violent reactivity, its functional groups have antibacterial effects. As a pure material, it can be harmful to human beings’ health when it gets into the respiratory system.
Ketones
Ketones are derived from secondary alcohols. In secondary alcohols, the hydroxyl group is attached to a carbon atom which is bonded to a second carbon atom. The functional group of ketones is composed of a carbon atom which is double bonded to a carbon atom and called the carbonyl group. In naming ketone compounds, the corresponding alkane’s name is taken first. Then the suffix -one is added. Description of the location of the carbonyl group is done with the help of numbers, before the name of the compound. The simplest ketone is a material with the common name acetone. Ketones form a homologous family of compounds.
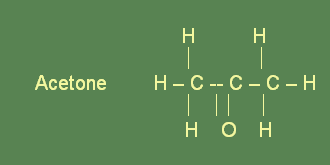
Significance and uses
Acetone is able to mix with water and ethanol in unlimited quantity. It is a very good solvent because
it can dissolve both polar and non-polar compounds. It is used often in industrial syntheses (in the production of plastic and some medicines), deriving its significance as an intermediate. In the household it is used as a nail polish remover. It is also used in the same way to dissolve and dilute some other types of paints.
Translating Finnish French Translations French Finnish Translating German Russian Translations Russian German Translating German
Translation Agency |
Email US |
Translation
Resources | Translation Jobs
Translation Agencies | World Languages | Translation Tips | Translation Services

Copyright © KENAX, by Karel Kosman - All Rights Reserved Worldwide.