
Translation Agency | Email US | Translation Resources | Translation Jobs | Translation Agencies
World Languages | Translation Tips | Translation Services
Translations of
Encyclopedia

Translation Agency |
Email US |
Translation
Resources | Translation Jobs | Translation Agencies
World Languages | Translation Tips | Translation Services
Carbon (C) is present in most compounds, both inorganic and organic. Carbon is fairly unreactive, but at high temperatures is forms compounds with
hydrogen, oxygen and various metals. Carbon is the only element with the ability to form chains and cyclical compounds of carbon atoms that line up next to each other in various lengths. This makes carbon the basis of organic chemistry. Thanks to carbon, more than 10 million known organisms survive, even thrive, on this Earth. In addition, there are around 200,000 known inorganic compounds which contain carbon.Carbon is an important rock-forming mineral, forming carbonates. As carbon dioxide (CO2), it can dissolve in water and is also found in the
atmosphere. It is an important component of all plants and animals, of all living organisms. Those organisms which died in the early years of our planet’s history have helped to create a huge supply of carbon and carbon-based fossil fuels, such as coal, oil and natural gas.In organic material which contains carbon, its atoms are bonded together in simple, single bonds (in saturated compounds) or in double and triple bonds (in unsaturated compounds). Carbon chains are the result. The sites which are not used for direct carbon-to-carbon bonding can be used for bonds with
hydrogen (hydrocarbons) or with other elements.According to the type of carbon chain present, we can differentiate between compounds with open chains (linear or branched – aliphatic or acyclic) and cyclic compounds. Aliphatic compounds are categorised in the ranks of branched carbon-containing compounds. Cyclical carbon-containing compounds are distinguished by their carbon atoms being arranged in a circle, in a closed cycle. Of these, the most important are aromatic carbon compounds, beginning with the founding member of the aromatic compounds,
benzene (C6H6). In it, carbon atoms form a circle together, with the individual bonds between them showing both single and double bond character, a sort of hybrid between the two. Some of the more important organic compounds are fats, proteins and hydrocarbons.Hydrocarbons
Hydrocarbons are composed exclusively of atoms of carbon and hydrogen. They are the simplest of all organic compounds. There are three types of
homologous families of hydrocarbons: alkanes, alkenes and alkynes. Alkanes contain only single bonds between carbon atoms. Alkenes contain at least one double bond. Alkynes contain at least one triple bond. Most of these types of hydrocarbons can exist with the same chemical formula in different form or chemical structure. When a compound has the same chemical formula but two possible structures, these two structures are called isomers.Hydrocarbon molecules can also contain what are called functional groups. These are groups which contain at least one atom which is neither carbon nor hydrogen. These functional groups can affect the chemical behaviour of the
molecule that contains them by giving that molecule special chemical properties. One example is ethanol – CH3CH2OH. Here, the functional group is –OH, with oxygen the determining atom.Stereochemistry
Stereochemistry is simply the three-dimensional arrangement of a molecule. Organic molecules of the same chemical formula can have their atoms arranged differently in space. When they do, they often have significantly different chemical properties.
Isomers are those types of compounds which have the same chemical formula but different atomic arrangements in space. Isomers can be divided into stereoisomers and structural isomers.
Stereoisometric molecules change their atomic arrangement as a result of changes in pressure or temperature. All bonds and types of bonds (single, double, triple) are conserved in the same original fashion, however.
Structural isomers have atoms which change their position in a molecule. One example is a linear compound (where all of the carbon atoms are lined up in linear fashion), compared to the same chemical formula compound with a shorter linear structure and branching (chain isomerism). Functional groups can change their position (functional isomerism), or can differ from another isomer in the position of a double or triple bond (bond isomerism).
The number of carbon atoms in a hydrocarbon determines how many forms that compound can take. The number of possible isomers in a compound rises as the number of carbon atoms it contains rises.
Translating Czech English Translations English Czech Translating Polish English Translations English Polish Translating French
Hydrocarbons are composed exclusively of oxygen and hydrogen. There are three types of homogeneous hydrocarbons (whose members differ by one CH2 unit): alkanes, alkenes and alkynes. The difference between these three groups is in the bond types between carbon. Alkanes form only single bonds, alkenes form double bonds, and in alkynes there is at least one triple bond.
The simplest alkane is methane. It is formed from one atom of carbon which is bonded with four atoms of hydrogen. If a CH
2 group is added, the second alkane compound is formed. The naming of alkanes, as with all other hydrocarbons, is based on the rules of IUPAC (International Union of Pure and Applied Chemistry). Alkane names all end with –ane (from alkan). In front of this ending is a prefix which describes the amount of carbon atoms, corresponding with either a Greek or Latin number. The first four alkanes are named according to historical convention.Methane: CH
4, ethane: C2H6, propane: C3H8, butane: C4H10, pentane: C5H12. The formula of all alkanes can be calculated according to the simple formula CnH2n+2. The number of carbon atoms is the defining factor as to which alkane is which. The alkanes, despite how many carbon atoms they contain, all share some common characteristics. For example, it is typical for all alkanes that they are not highly reactive, they burn well, and they react analogously with halogens in photochemical substitution reactions (exchange reactions). With increasing size of the molecule in the alkane family, alkanes begin to differ from one another in a fundamental way. The first four alkanes are found in the gaseous state of matter. Alkanes containing 5-16 carbon atoms are liquids, and alkanes with 17 or more carbon atoms are solids. Boiling and melting points rise with increasing atomic number.Branched alkanes are first named according to the amount of carbon atoms they contain in a row. If a radical is contained in an alkaline compound, the -ane ending is replaced by -yl. The branch must be denoted in some way, so as to pinpoint its location on the main carbon chain. For this reason, carbon atoms are numbered from left to right from least to greatest number, so that the branch is arbitrarily assigned the lowest number possible. The main chain has to be the longest one in the molecule. If there are multiple chains in the molecule, they are assigned letters of the alphabet.
The bond between carbon and hydrogen in an alkane molecule is a weak, polar
atomic bond. For this reason, the individual atoms of alkanes carry only a very weak partial charge. These partial charges cancel each other out over the molecule, since it is perfectly symmetrical. The result is a molecule which is non-polar overall. This is not to say one molecule of an alkane does not interact electrostatically with other atoms of its own kind. Weak van der Waals intermolecular forces are found between non-poplar molecules, causing them to mutually attract and repel each other in a weak way. The size of these forces increases as molecule size increases. According to this idea, the characteristics of unbranched alkanes change with increasing size of the carbon chain.At room temperature, the first four alkanes are found in the gaseous
state of matter. Pentane is the first of the liquid alkanes. Until hexane (16), alkane compounds become more and more viscous (parafin oil), because their viscosity rises as the strength of van der Waals forces increases. From heptadecane (17), the alkanes are solids (parafins). Their melting and boiling points rise as a function of the number of carbons in their chains.Alkanes burn readily. When they do burn,
carbon dioxide and water are the products. With increasing chain size, alkanes, given the same amount of oxygen, burn less easily, so that more carbon soot (elementary carbon) is formed with increasing chain size. In alkane molecules, all bonds are said to be saturated. For this reason, alkanes are not very reactive. They do tend to form compounds with halogens.Because molecules carry a partial charge, there are forces and attractions between neighbouring molecules. These forces between molecules are very small, but they are big enough to hold the molecule together. The longer the carbon chain of a molecule, the more atoms can take part in these mutual forces, and the greater the resultant attractive force. If the inner forces in smaller alkanes are small, they may not be strong enough to hold the molecule together at room temperature. With increasing carbon chain size, however, these intramolecular forces do increase. At a chain length of 17 carbon atoms, the van der Waals forces are so strong that the individual molecules are held together in the solid state of matter.
Alkenes (olefíns) are unsaturated compounds of carbon with hydrogen which contain one or two double bonds between atoms of carbon. They burn to form carbon soot and carbon dioxide and water. They are more reactive than alkanes because of the fact that they contain double bonds.
Multiple bonds (double, triple bonds) are energetically less advantageous for atoms than corresponding single bonds. For this reason, the atoms in a compound will attempt to break multiple bonds to form single bonds, which are more advantageous energetically. This explains why compounds which contain double and triple bonds are so much more reactive than those which contain single bonds. The alkenes include ethene: C
2H4, propene: C3H6, butene: C4H8 and pentene: C5H10. Up to butene, the alkenes occur as gases. Up to hexadecene (C16H32) they are liquids, with higher alkenes found in the solid state of matter. Their general chemical formula is CnH2n.Alkynes (acetylenes) are unsaturated necyclical hydrocarbons which contain one or more triple bonds between atoms of carbon. When they burn, they tend to form carbon soot. When oxygen is present during burning, high temperatures can be reached. The general formula for alkynes is C
nH2a-2. Among these are acetylene: C2H2, propyne: C3H4 and butyne:C4H6.The carbon atoms of hydrocarbons can be arranged in circles. These cyclical hydrocarbons with single bonds are called cycloalkanes. Benzene and its derivatives, however, are called aromatic hydrocarbons. They contain double bonds. Benzene (first called benzol) was discovered in 1825 by M. Faraday. The name benzol was coined by J. von Liebig. Because benzene is not an
alcohol, we call it benzene, not benzol. Benzene is a colourless liquid which refracts light and has an aromatic odour. This characteristic smell was the reason why benzene’s group is called the aromatic compounds. Benzene is less dense than water and does not mix with water. On the other hand, it does mix with, or dissolve in, non-polar solvents. Benzene can itself dissolve fats, resins and rubber. Its boiling point is 80.1° C, lower than that of water. At 5-6° C, benzene solidifies and begins to crystallise. When it is burned, benzene releases carbon soot. In its pure form, benzene can be dangerous for human health. If humans are exposed to benzene for long periods of time, their livers, kidneys and bone marrow can be harmed. Benzene is a carcinogen, but it is a useful material in chemistry, serving as a reactant in the synthesis of a number of organic compounds.Cyclic hydrocarbons can be differentiated from aliphatic hydrocarbons. The cycloalkanes, which are composed of multiple CH
2 groups and have no double bonds, form a homologous group of compounds. The first member is cyclopentane. The same as the next member cyclohexane, it is very unstable. Because cycloalkanes are saturated compounds, they, like linear alkanes, are not very reactive. They also share a number of properties. The aromatic hydrocarbons are derived from benzene. Group members have six free valence electrons which are distributed in a circle in the form of a charged cloud. Because of the presence of these valence electrons, we can predict that the reactivity of these aromatic compounds will be similar to other unsaturated hydrocarbons. This time, however, our prediction is incorrect: Benzene is much less reactive than other unsaturated hydrocarbons. Only at high temperatures and in the presence of a catalyst can benzene take on another hydrogen atom. When it does, cyclohexane is the resultant product.Benzene (benzol), which was discovered as early as 1825, was described by A.F. Kekule von Stradonitz for the first time in 1865. According to Kekule’s description, benzene was a circular compound with six atoms of carbon. The benzene circle contained three double bonds which alternate with three single bonds. Kekule believed that these double bonds were fixed in one place in the molecule. He thought that there were two isomeres of benzene which existed side-by-side.
Modern models of benzene’s structure show that each carbon atom has associated with it one unpaired electron, a free electron. These unpaired electrons are divided among the circle in the form of a charged cloud. They do not have one certain position in the formation of double bonds. This strange electron arrangement is called mezomeric. It is the reason why benzene is not as reactive as we might expect as compared to other compounds which contain double bonds.
Cyclohexane belongs to the cyclic hydrocarbon family of single-bonded compounds between carbon atoms. It is made of six carbons, each having two hydrogens associated with it.
Translating German French Translations French German Translating Polish German Translations English French Translating Polish
The noble gases are found in Group VIII of the
main group elements, the A groups. They have a full outermost electron shell and are therefore nearly unreactive. The lighter noble gases do not form compounds at all, and the heavier ones form very few, these being able to be formed and exist only under certain conditions. The elements of the noble gas group include: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Ra). All occur in the gaseous phase of matter. It is possible to produce them through the distillation of condensed air (at temperatures of around -200° C).The noble gases are not flammable. Helium is used in hot air balloons and other balloons, because it is lighter than air. Radon is the product of the fission reaction of the radioactive element radium. The other noble gases are used in numerous types of lighting because they do not react (light bulbs, neon tubes).
Halogens are found in the seventh main group of elements. They have seven electrons in their outermost electron shell. They can react with other elements and form covalent bonds as well as being able to react to form ionic bonds. They occur in nature in compounds. Smaller halogens, the ones at the top of the periodic table, are more reactive than the halogens in the lower portion of the table, so the smaller halogens can take the place of larger ones in compounds, replacing them or substituting for them. All halogens are poisonous. The halogens are: fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). Fluorine and chlorine are
gases at room temperature. Fluorine corrodes and attacks almost all other materials, including glass. Chlorine is highly poisonous. Other halogens are either liquids or solids at room temperature, based on their size, where the largest halogens are solids. In the gaseous form all halogens are highly poisonous.Substitution generally
The substitution of halogens with alkanes is another way besides burning that they can react. In a substitution reaction, one atom of hydrogen is replaced by one atom of a halogen. This type of reaction is called a halogenation. The halogenation of alkanes occurs in the presence of light, making it a photochemical reaction.
Methane (C
2H4) reacts with chlorine (which occurs as a two-atom molecule Cl2) in the presence of light to produce methyl chloride, CH3Cl, and hydrogen chloride (HCl).These compounds can be differentiated according to various criteria, including:
1. The type of halogen, for example fluoro-, chloro-, bromo-, and iodo-.
2. The type of carbon chain: open, closed, aromatic, saturated, unsaturated.
3. The number of atoms in the halogen: mono-, di- and poly halogen compounds.
The name of the compound is based on the number of carbon atoms present, and where the substitution of a halogen for a hydrogen atom has taken place. Before the name of the hydrocarbon the names of the substitued halogens are given, in alphabetical order if possible. Each carbon atom is assigned a number so as to place the substituted halogen at as low a number as possible. Then the number of the carbon which has been substituted is placed before the halogen prefix. For example:
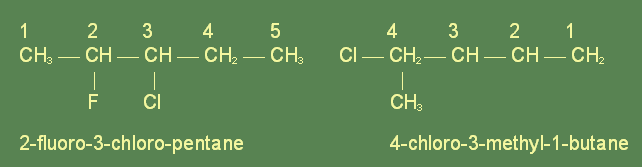
The carbon chain is always numbered in such a way so that the substituting groups are assigned the lowest numbers. If, however, there are multiple substitutions or some larger group has been substituted, a
functional group, that is, it is assigned the lowest possible number.Fluorine is the first of the halogen group, which means that it is able to substitute for all of the other halogens in a chemical bond. For this reason, hydrocarbons containing fluorine are very stable, non-flammable, and are not poisonous. They are used as an ingredient in aerosol sprays or as the refrigerant liquid in refrigerators, and as a solvent. Their use has become less popular in recent years because of the damage they do in the atmosphere to the
ozone layer.Translating Turkish Swedish Translations Swedish Turkish Translating Greek Dutch Translations Dutch Greek Translating French
|
Fossil fuels and many other raw materials are the remains of plants and animals which lived millions of years ago. They are composed of hydrocarbons, so we call them fossil carbon compounds. Fossil fuels and other raw materials can occur as solids, usually in stratified layers of earth. Or, these materials can be liquids, as with oil. Natural gas, which is found in underground caves in often great quantities, occurs sometimes in kilometre-wide supply. Because coal is usually deposited near the surface of the Earth, it was the first fossil fuel to be used as an energy provider by our distant ancestors. Oil and natural gas have gained their importance as fuels mostly as raw materials for industry, and that in more recent times. Coal is a flammable material made from the remains of plants and other organic substances, which during millions of years of geological history has been charred, or carbonised, to a brown or black colour in a sedimentary layer. Coal can be divided into various kinds based on its degree of carbonisation: brown, black and anthracite. While brown coal contains about 60-70% carbon and has a relatively high water content, with ash matter and bitumen making up around (asi 7-20% ), hard, black coal is much richer in carbon (75-92% ), while being lower in water content, ash and bitumen. This makes it more expensive, of course. Anthracite is coal with a high degree of carbonisation (containing more than 91.5% carbon). Its water and ash content are negligible. Carbonisation, or charring, is a process by which fossilised compounds become coal. In the process of this long-term geochemical change, plant material (cellulose and lignite) are transformed into peat, then to brown coal, hard coal and anthracite. One of the prerequisites for carbonisation is a large amount of matter containing carbon. A damp environment is also needed, as is a moderate climate. The organic material has to be covered with a thick layer of mineral sediment (in a depression in the earth). Carbonisation begins at high temperature and pressure in the absence of air, leading to the decrease of the concentrations of hydrogen and oxygen in the material. On the other hand, the relative concentration of carbon in the material increases.The degree of carbonisation in a material rises from peat material, to brown coal, to black coal, to anthracite. The various types of coal, with their differing degrees of carbonisation, have variable heat, or calorific, value, with the least amount in peat. Calorific value is the amount of heat energy which is released when 1 kg of material is burned. The calorific value of the individual fossil fuels is given in units of kilojoules per kilogram (KJ/kg) wood 16 800 KJ/kg |
|
Coal is used in a number of industrial processes, and in households, mostly as a fuel.
Natural gas is a naturally-occurring gas which is often found in caverns in the earth, often together with oil. Natural gas can also be found in porous layers of sand. Natural gas is a mixture of gases composed mostly of methane. Other gases which can be found in natural gas are other hydrocarbons (ethane, propane, butane), nitrogen, hydrogen, water and helium. It is formed by the transformation of fossil compounds by long geological processes. Natural gas can be won by digging natural gas wells. Natural gas need not be taken from the surface of the earth, because the internal pressure of the layer where it is found is sufficient to push it up to the surface of the earth. It is used today as a propulsion and heating material. It is also widely used in many chemical processes.
Translating Swedish Italian Translations Italian Swedish Translating Arab German Translations German Arab Translating Czech
Composition and uses of oil and natural gas
Oil begins to be formed when microorganisms decompose plant and animal matter in the absence of air. This previously organic material is amassed thousands of meters below the surface of the Earth, in porous caverns in sedimentary rock formations. Oil is a brown to black liquid. It is more or less viscous, thanks to its chemical composition. Oil is a mixture of materials, but it is primarily composed of normal or cyclic hydrocarbons (up to 80-90
% ) and water (10-14% ). Oil can be burned when it is in its liquid state, as a heating oil, but it is also a raw material in the production of other fuels, lubricants, paraffins and bitumens. Besides these uses, it is a geochemical raw material which is used in other special processes which produce various powders, plastics and synthetic fibres, among others.Separation of individual oil fractions using distillation
Oil is composed of a mixture of linear and cyclic hydrocarbons. These can be separated by taking advantage of their different
boiling points, using distillation to separate the components. When oil is heated, its fractions, or components, with the lowest boiling point vaporise first. If the distillation is carried out gradually and slowly, a fraction can be cooled, and the gas will condense. In this way, one liquid fraction can be isolated, and this fraction should be fairly pure. This separation of the individual fractions of oil is called a fractional distillation. Of course, each individual fraction separated contains a mixture of materials. Each mixture has its own special uses as well.Approximate boiling point (° C) Fraction Uses
< 30 gaseous heating oil
30-200 gasoline, petrol motor oil, solvents
150-240 petrol solvents, kerosene, heating oil
200-370 diesel oil diesel oil, heating oil
350-500 lubricants lubricants, greases
> 500 bitumen asphalt products, freeway materials
From a practical point of view, fractional distillation means that when oil is heated, it vaporises, its fumes rising up a column from its liquid origin in the bottom of the column to the top. These fumes are horizontally divided into individual columns. The temperature of each fraction decreases as the column rises away from the original liquid material. The fractions condense and can be divided out. Before a fractional distillation of oil is carried out, the original oil sample must be cleaned, which means removing the salts it contains, and removing any water which might be present.
Mineral oils and motor oil
Fuels contain alkanes and cyclic hydrocarbons in their liquid form, with two main groups which can be distinguished. Motor oils are products with low boiling points (30-200° C). These can be used in the propulsion systems of Otto motors. Diesel oil is separated from a boiling fraction at temperatures of 200-300° C. It is used in diesel motors and has a higher burning temperature than petrol. When the fuel is completely burned, water and
carbon dioxide are produced. If there is insufficient oxygen available during the burning process, carbon monoxide, a very poisonous and reactive gas, is produced. The release of excessive carbon monoxide into the air can result in problems in the atmosphere. To help solve this problem, modern cars are equipped with catalytic converters, in order to decrease the amount of carbon monoxide and other harmful gases into the atmosphere. Catalytic converters change carbon monoxide into the less harmful carbon dioxide, as well as transforming harmful nitrogen oxides into less harmful compounds.Environmental consequences of burning fossil fuels
Besides water, carbon dioxide is formed when fossil fuels are burned. This gas is released into the atmosphere. Atmospheric concentration of carbon dioxide rises, and the result is the we have to be concerned about possible changes to the Earth’s climate. Carbon dioxide is not the only harmful gas released in burning reactions: Nitrogen-containing oxides and sulphur oxides are also released. The latter are easily dissolved in water, which makes them a main ingredient in rain, which falls on both land and water, acidifying our environment and bringing even more consequences to bear. The burning of fossil carbon sources, then, has significant effects on the Earth’s climate, nature, agriculture and of course, our health. The use of alternative sources of energy, therefore, is the only possible answer to this dilemma. Only in this way can the negative effects of the release of these harmful gases be limited. Another possibility, of course, is using less energy, especially that derived from fossil fuels.
The significance of the above-mentioned alternative sources of energy has grown and continues to grow, on the one hand because of the pollution of our environment which is the result of the burning of fossil fuels. Not to be underestimated, however, is the reasoning that the stocks of these fuels are bound to be used up at some point in the not so distant future. Fossil fuels are one type of exhaustible energy source. There is only a certain amount of fossil fuels found on Earth; once this amount is gone, there is no way to produce more. As these stocks are used up, it will become more and more important to generate the energy humanity needs from alternative sources which can be regenerated and can therefore be used in a practically unlimited way. Wind energy, solar energy, hydroelectric energy and geothermic energy are only some of the possibilities.
Translating English Polish Translations Polish English Translating Hungarian Norwegian Translations Norwegian Hungarian Translating Swedish
Nomenclature of carboxylic acids
Carboxylic acids are organic
acids which contain a functional carboxylic group (-COOH). Aliphatic, saturated monocarboxylic acids which contain one carboxylic group in the molecule form a homologous group with the general chemical formula (CnH2a+1COOH). In naming of these acids, the compound name of the corresponding alkane is taken as an adjective, with the name of the acid added on. The first acids in the homologous group carry their traditional names:formic acid (HCOOH)
acetic acid (CH3COOH)
propionate acid (CH3CH2COOH)
butyl acid (CH3CH2CH2COOH)
When the
salts of carboxylic acids are named, the base name of the corresponding alkane is taken and an -ate suffix is added to the end. The cation of the salt is indicated by an adjective of the name of the element.The hydrocarbon portion of the molecule of carboxylic acids is named according to how the corresponding alkane would be named. This is characterised by the main symbol of the carboxylic acid - for example the carboxylic group (-COOH), which is the functional group.
Physical properties and reactivity
Molecules of carboxylic acid are polar. The carboxylic groups contained on the molecules form
hydrogen bonds with neighbouring molecules. Thanks to these weak intermolecular forces, carboxylic acids have high melting and boiling points. With increasing size of the hydrocarbon rest of the molecule, the polar character of the functional group is masked by the non-polar character of the hydrocarbon chain. The first homologous members of the series are liquids which are soluble in water. As the series continues, however, its members become solid at room temperature, and begin dissolving in non-polar solvents. Because of their acidic character, carboxylic acids form salts with impure metals.The acid reactions which take place among carboxylic acids are caused to a great degree by the presence of their carboxylic group. Its oxygen atom increases the polar character of bonds formed with oxygen and hydrogen, making it very easy for it to release an electron - to form a carboxylic ion.
Acetic acid
Acetic acid is the base ingredient of common table vinegar. Acetic acid is a clear liquid with an acrid odour. It is corrosive, and combined with indicators, it reacts in an acidic manner. Concentrated acetic acid is known as icy acetic acid, because below 16.6° C, its melting point, it hardens into a metal-like structure. Acetic acid is easily soluble in water and ethanol. When it is reacted with impure
metals, hydrogen, metal salts and acetic acids known as octanes (acetates) are formed. Acetic acid is used in the food service industry. It is used as a preservative in the production of some groceries.In industry, acetic acids are used to produce acetic acid esters. These are good solvents. In addition, acetic acids can be used in the production of plastics, artificial silks, some medicines and paints and other colourings.
The acids which are found in vinegar are produced with the help of the bacteria found in alcoholic wine. This reaction has as its mechanism the oxidation of ethanol by oxygen contained in the air, to acetic acid. This process is called acetic fermentation.
bacteria
CH
3CH2OH + O2 ¾ ¾ ¾ ® CH3COOH + H2OEthanol acetic acid
Production of esters and their reactivity
Esters are produced when carboxylic acids are allowed to react with alcohols. Esters have the functional group -COO-. Water is also produced as a by-product in the reaction which produces esters. This is a reversible reaction, sometimes also called an equilibrium reaction. The break-up of an ester (reversible reaction) is called the hydrolysis of an ester. The synthesis of an ester is a multi-step reaction, with additions and eliminations included.
Significance of esters
One of esters’ most distinguishing characteristics is their intense odour, similar in some cases to fruits and other plants. For this reason, they are often used in the food service industry, especially in the production of certain delicacies or additives which tend to intensify some tastes in foods. Esters are good solvents. They are ingredients in some glues and paints. In the production of an ester from an acid derived from an alkane bonded to glycerin (glycerol), fats and oils are produced. The oils contain
unsaturated carboxylic acids of ester glycerin. The fats contain saturated acids. Waxes are esters of higher aliphatic alkanols with carboxylic acids. They have 16-32 carbon atoms in each molecule. Fats and oils are very significant for living organisms, because important materials necessary for living systems can dissolve in them.
Glycerol – an ingredient in fats and oils
Fats and oils are esters of glycerine and carboxylic acids. Glycerin carries the systematic chemical name propantriol. The carbon chain is derived from that of propane. One hydroxylic group is bonded to each carbon atom.
Translating German Swedish Translations Swedish German Translating French Rumanian Translations Rumanian French Translating Rumanian
|
Share the knowledge: |
Translation Agency |
Email US |
Translation
Resources | Translation Jobs
Translation Agencies | World Languages | Translation Tips | Translation Services

Copyright © KENAX, by Karel Kosman - All Rights Reserved Worldwide.